Adalimumab-fkjp Clinical Profile
Built with patients in mind
With the easy-to-use 2-click Adalimumab-fkjp prefilled pen, starting treatment is as simple as “click, click, go!”1
No buttons to push—patients simply remove the cap and push the device against the skin to trigger their injection.2
Adalimumab-fkjp is also available in 20 mg/0.4 mL and 40 mg/0.8 mL prefilled syringes.

Protective Cap
Covers needle for patient safety

Citrate Free
Minimizes injection site pain

Viewing Window
Shows progress of injection

2 Audible Clicks
Confirms start and end of injection

Plastic Design
Reduces breakage concerns

Made Without Natural Rubber Latex
Reduces hypersensitivity reactions
Keep on treating with Adalimumab-fkjp
Adalimumab-fkjp is a recombinant human IgG1 monoclonal antibody (mAb) that blocks TNF-α.2 Sound familiar? Because Adalimumab-fkjp works the same way as Humira, you can continue helping your patients manage a wide range of autoimmune and inflammatory conditions.2
Adalimumab-fkjp Indications*
Moderate to Severe chronic
Plaque Psoriasis
in adults
Moderate to Severe
Ulcerative Colitis
in adults
ACTIVE
Ankylosing Spondylitis
in adults
Moderate to Severe
Rheumatoid Arthritis
in adults
Moderate to Severe
Crohn’s Disease
in adults and children 6 years and older
Moderate to Severe
Hidradenitis Suppurativa
in adults
MODERATE TO SEVERE POLYARTICULAR
Juvenile Idiopathic Arthritis
in children 2 years and older
NON-INFECTIOUS
Uveitis
in adults
ACTIVE
Psoriatic Arthritis
in adults
Biosimilars aren’t just what we do—they’re all we do
Each year, biosimilars save the US healthcare system over $5 billion.*3 By expanding the availability of high-quality biosimilars such as Adalimumab-fkjp here and around the world, Biocon Biologics can make a meaningful difference for patients and those charged with their care.
Robust Pipeline
More than 20 biosimilars approved or in development
Strong Portfolio
9 biosimilars launched across global markets
Wide Impact
5.5 million patients served in over 100 countries
*Estimates of reductions in direct spending on biologic drugs from 2014 through 2024.
The proof behind Adalimumab-fkjp
Adalimumab-fkjp has achieved all the benchmarks for biosimilarity to Humira.
The totality of evidence speaks for itself.1-3
Adalimumab-fkjp meets the rigorous biosimilar approval standards of the FDA
- An application for a proposed biosimilar generally must include information showing that the proposed product is biosimilar to a reference product3
- The process typically requires analytical studies, nonclinical (animal) studies, clinical pharmacology (pharmacokinetics), and one or more clinical studies3
- For more information about the FDA approval process, please visit the FDA biosimilars review and approval page
PD=pharmacodynamic; PK=pharmacokinetic.
Extrapolation
Totality of Evidence
+
Scientific Justification
=
Extrapolation to Additional Indications
Adalimumab-fkjp has 2-year switching data
The comparative clinical data required for FDA biosimilar approval were evaluated for an extended period—including the effects of single- and double-switching treatment in a defined population.3
- There was no long-term clinically meaningful difference between Adalimumab-fkjp and Humira with respect to safety, efficacy, and immunogenicity3
- There was no impact of switching and double switching between the 2 drugs3
Click below to expand and view the evidence.
Study Design
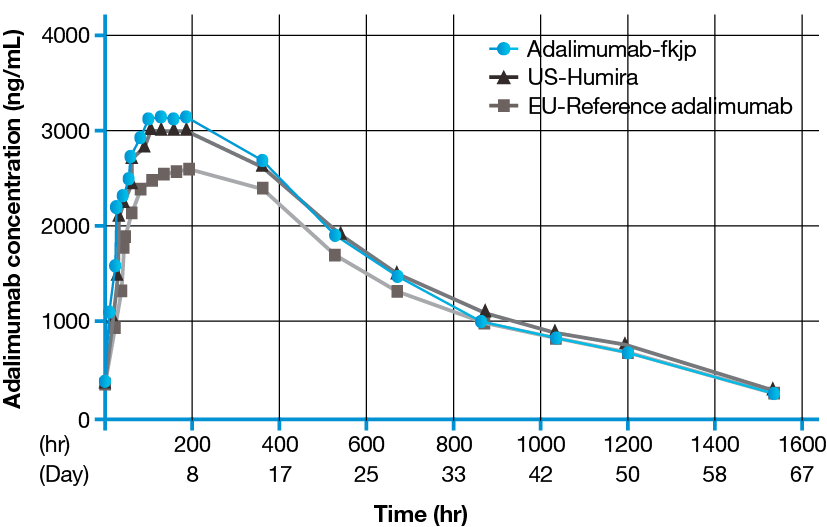
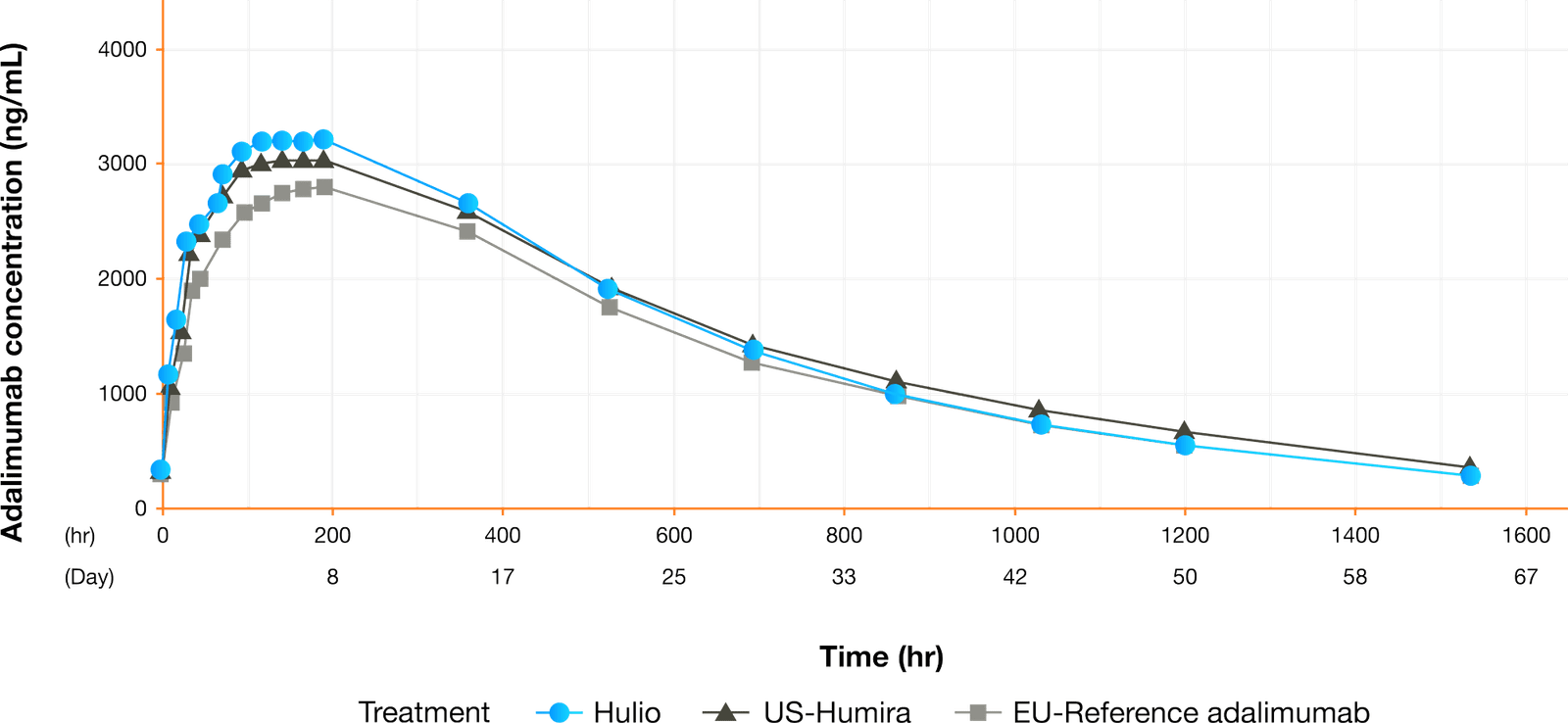
Results from a Phase I, randomized, double-blind, parallel-group study compared the pharmacokinetic profile of the Biocon Biologic’s biosimilar adalimumab, Adalimumab-fkjp, with reference adalimumab sourced from the European Union and the US. A total of 180 healthy subjects (170 men and 10 women of non-childbearing potential) were enrolled.1
Concentration vs Time
- Adalimumab-fkjp has a PK profile similar to EU-approved and US-licensed adalimumab reference products1
- Adalimumab-fkjp was well tolerated by healthy volunteers in this Phase I PK study, with a safety profile similar to that of its reference product3
EU=European Union
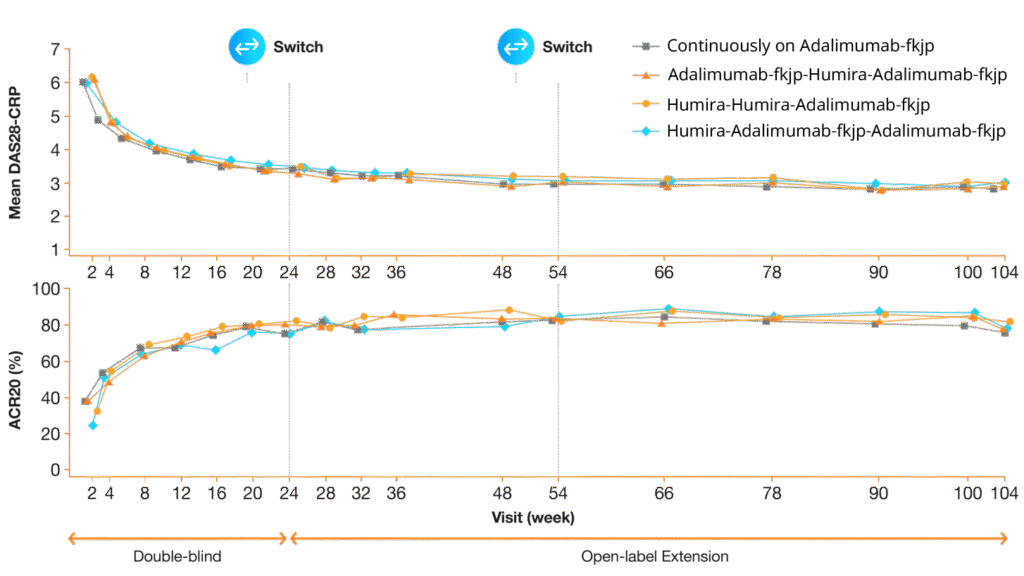
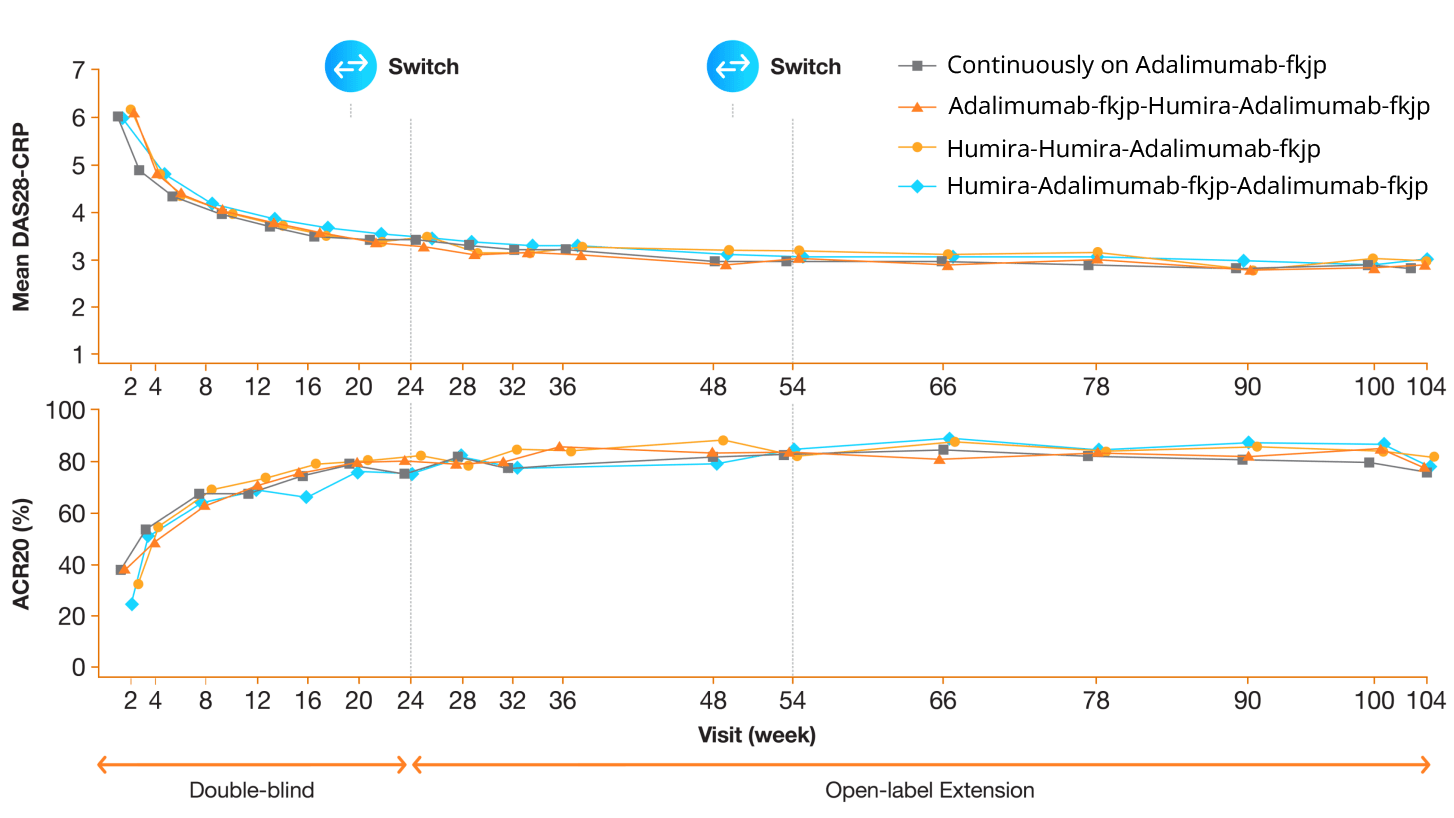
Efficacy highly similar during double-blind phase2,3
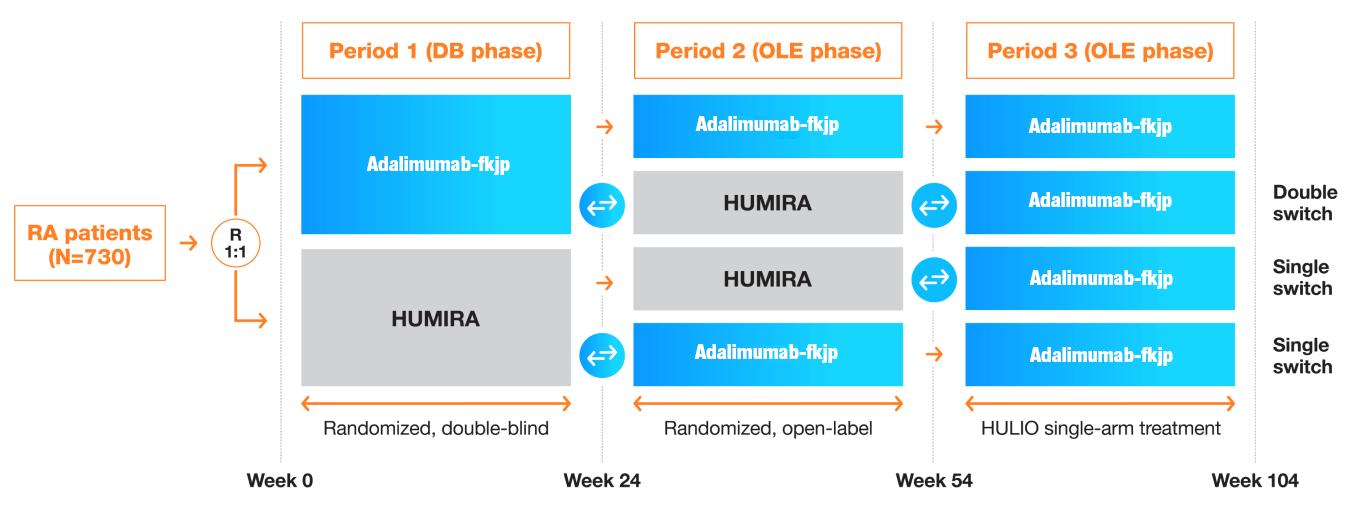
Study Design
A randomized, double-blind (DB), phase 3, 24-week study compared the efficacy, safety, and immunogenicity of Adalimumab-fkjp with Humira in patients with moderate-to-severe rheumatoid arthritis (RA) inadequately controlled with methotrexate. Patients who completed the DB study were enrolled in the open-label extension (OLE) study and re-randomized 2:1 to receive Adalimumab-fkjp or Humira; two-thirds continued on the same treatment and one-third switched for 30 weeks. Then all patients received Adalimumab-fkjp for a total duration of 2 years.2,3
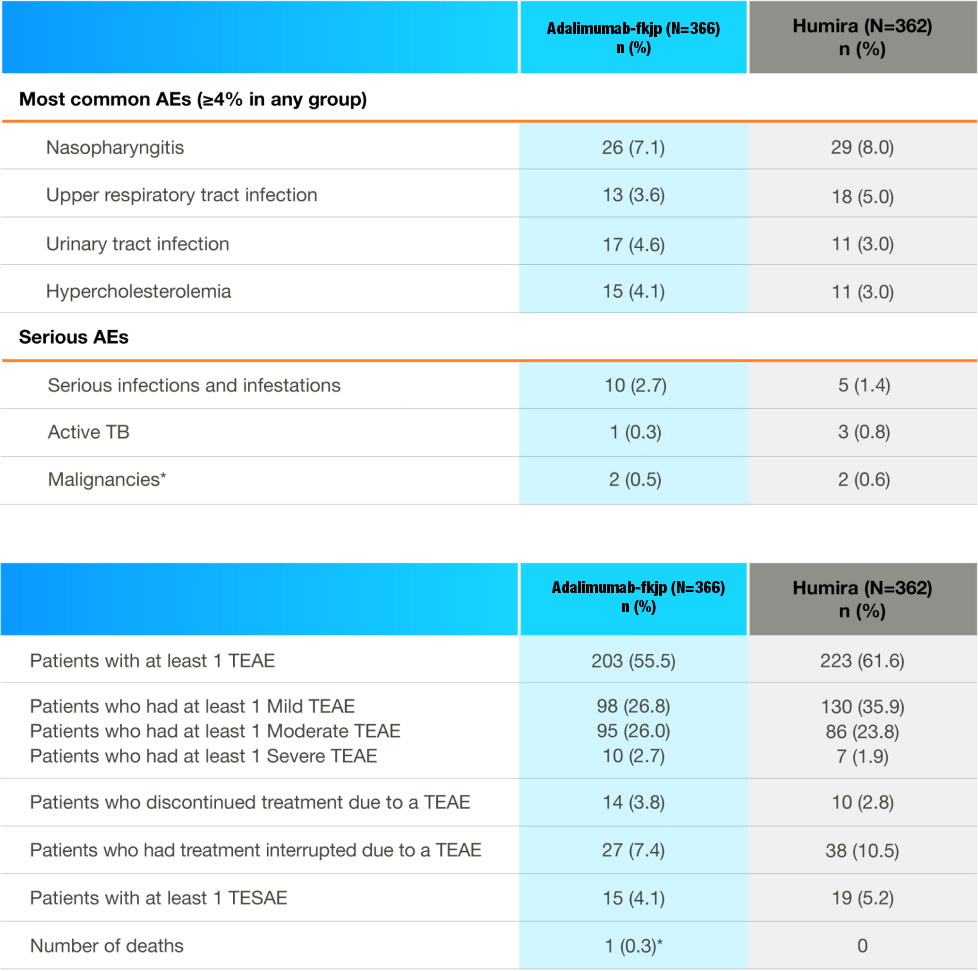
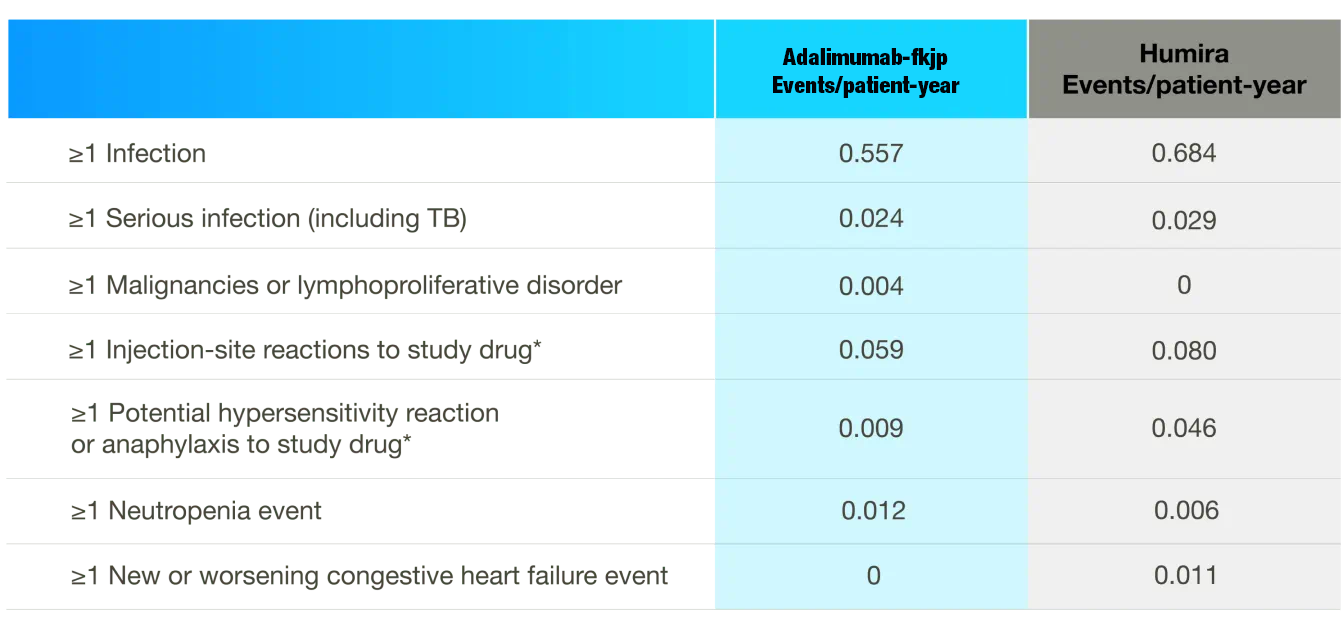
Safety profile highly similar during double-blind phase2,3
Adverse Reactions at Week 24
- Data shown refer to the same 24-week, double-blind trial as the efficacy data; please see the study design under the efficacy section
- Adalimumab-fkjp and the adalimumab reference product have similar clinical safety profiles2
AEs=adverse events; TB=tuberculosis;
TEAE=treatment-emergent adverse event;
TESAE=treatment-emergent serious adverse event.
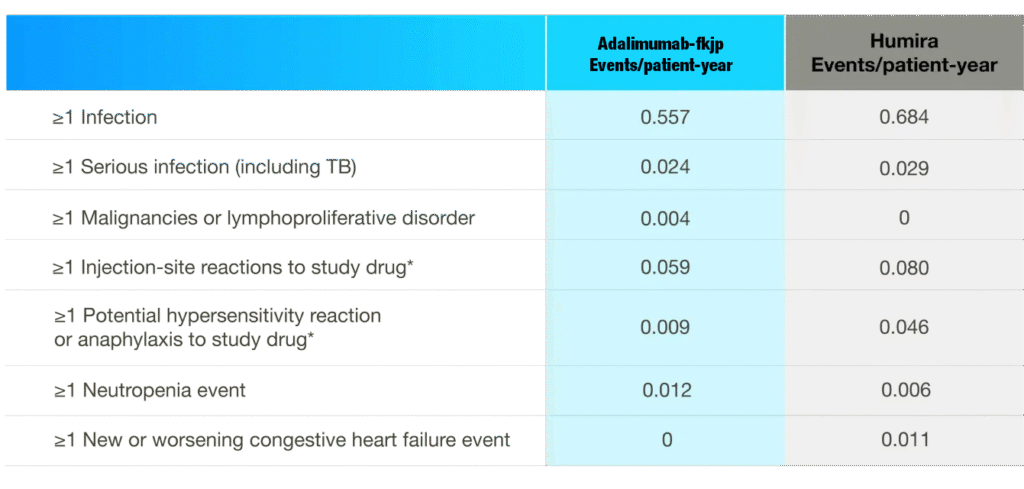
Summary of Treatment-emergent Adverse Events of Interest During 2-year Study
- The Adalimumab-fkjp and Humira analysis included 673.7 patient-years and 175.4 patient-years, respectively3
- Safety of Adalimumab-fkjp was comparable to Humira and maintained over long-term treatment during the open-label extension study, even when patients were switched between Adalimumab-fkjp and Humira3
- Please see the study design under the efficacy section
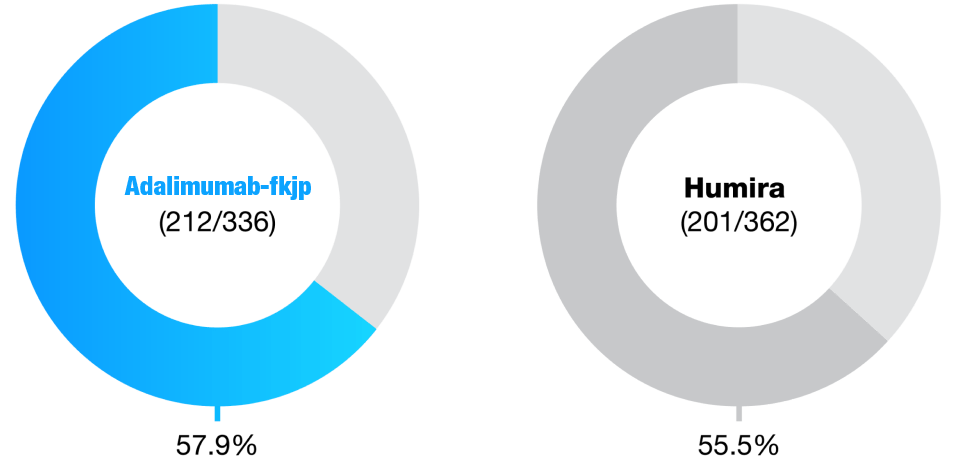
Immunogenicity highly similar during double-blind phase2
Anti-Drug Antibodies at Week 24
- Data shown refer to the same 24-week, double-blind trial as the efficacy data; please see the study design under the efficacy section
- Proportion of patients with anti-drug antibodies (ADA+) was similar between Adalimumab-fkjp and Humira groups at Week 242
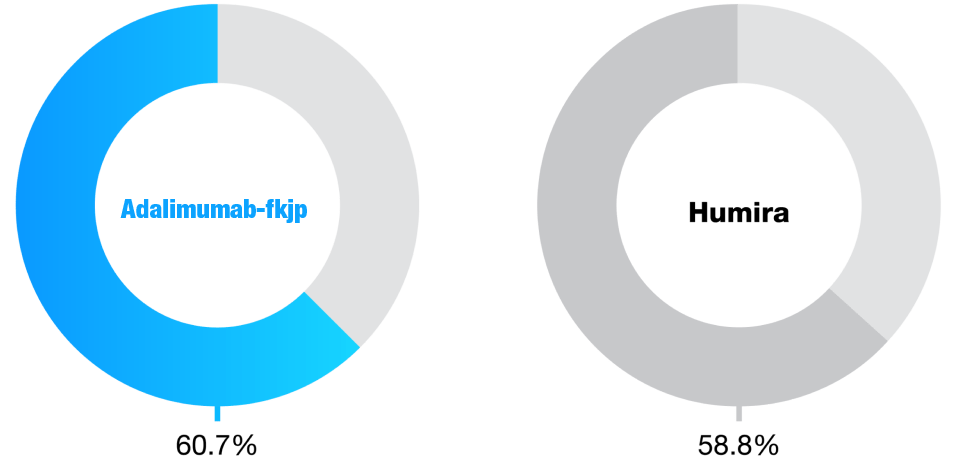
Anti-Drug Antibodies During 2-year Study
Adalimumab-fkjp keeps it familiar
Especially the dosing you’ve come to expect with Humira.1,2
Administering Adalimumab-fkjp
Considerations
- Dosing is similar to Humira—no new doses or dosing schedule to learn1,2
- Adalimumab-fkjp is intended for use under the guidance and supervision of a physician. A patient may self-inject Adalimumab-fkjp or a caregiver may inject Adalimumab-fkjp using either the Adalimumab-fkjp Pen or prefilled syringe if a physician determines that it is appropriate, after proper training in subcutaneous injection technique1
- Prior to initiating Adalimumab-fkjp and periodically during therapy, evaluate patients for active tuberculosis and test for latent infection1
- If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regular scheduled time1
- There is no dosage form of Adalimumab-fkjp that allows weight-based dosing for pediatric patients weighing less than 15 kg (or 33 lb)1
- Your patients may receive Adalimumab-fkjp injection
Dosage Forms and Strengths

Prefilled Pen
- Injection: 40 mg/0.8 mL in a single-dose pen
- 2-step process with audible clicks and no buttons assures correct use, even for patients with disabilities
- Does not need to be mixed or prepared
- Medicine is injected automatically; patients never see the needle
Prefilled Syringe
- Injection: 40 mg/0.8 mL in a single-dose prefilled plastic syringe
- Injection: 20 mg/0.4 mL in a single-dose prefilled plastic syringe
Adalimumab-fkjp helps you get going and keep going
See how similar transitioning to Adalimumab-fkjp will be for your current Humira patients.1,2
Click on the therapeutic area below to reveal Adalimumab-fkjp-specific dosing for each indication.
ADULTS
MAINTENANCE
ADULTS
40 mg/0.8 mL
every other week
Indication: Adalimumab-fkjp is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active rheumatoid arthritis. Adalimumab-fkjp can be used alone or in combination with methotrexate or other non-biologic disease-modifying anti-rheumatic drugs (DMARDs).1
ADULTS
MAINTENANCE
ADULTS
40 mg/0.8 mL
every other week
Indication: Adalimumab-fkjp is indicated for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active psoriatic arthritis. Adalimumab-fkjp can be used alone or in combination with non-biologic DMARDs.1
ADULTS
MAINTENANCE
ADULTS
40 mg/0.8 mL
every other week
Indication: Adalimumab-fkjp is indicated for reducing signs and symptoms in adult patients with active ankylosing spondylitis.1
MAINTENANCE
15 kg (33 lbs) to
<30 kg (66 lbs)
20 mg/0.4 mL
every other week
≥30 kg (66 lbs)
40 mg/0.8 mL
every other week
<30 kg (66 lbs)
MAINTENANCE
ADULTS
20 mg/0.4 mL
every other week
40 mg/0.8 mL
every other week
There is no dosage form for Adalimumab-fkjp that allows weight-based dosing for pediatric patients below 15 kg.
Adalimumab products have not been studied in patients with polyarticular juvenile idiopathic arthritis less than 2 years of age or in patients with a weight below 10 kg.
Indication: Adalimumab-fkjp is indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis in patients 2 years of age and older. Adalimumab-fkjp can be used alone or in combination with methotrexate.1
ADULTS
INDUCTION
ADULTS
Day 1:
80 mg/0.8 mL
MAINTENANCE
ADULTS
Day 8:
40 mg/0.8 mL
every other week
Indication: Indication: Adalimumab-fkjp is indicated for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active psoriatic arthritis. Adalimumab-fkjp can be used alone or in combination with non-biologic DMARDs.1
ADULTS
MAINTENANCE
ADULTS
40 mg/0.8 mL
every other week
Indication: Adalimumab-fkjp is indicated for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active psoriatic arthritis. Adalimumab-fkjp can be used alone or in combination with non-biologic DMARDs.1
ADULTS
INDUCTION
ADULTS
Day 1:
80 mg/0.8 mL
MAINTENANCE
ADULTS
Day 8:
40 mg/0.8 mL
every other week
Current adalimumab patients do not need to repeat induction dose—initiate Adalimumab-fkjp as next scheduled dose.
Indication: Adalimumab-fkjp is indicated for the treatment of adult patients with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy, and when other systemic therapies are medically less appropriate. Adalimumab-fkjp should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician.1
ADULTS
INDUCTION
ADULTS
Day 1:
160 mg/0.8 mL*
>
Day 15:
80 mg/0.8 mL
MAINTENANCE
ADULTS
Day 29:
40 mg/0.8 mL
every week
or
80 mg/0.8 mL
every other week
Current adalimumab patients do not need to repeat induction dose—initiate Adalimumab-fkjp as next scheduled dose.
*Given in 1 day or split over 2 consecutive days.
Indication: Adalimumab-fkjp is indicated for the treatment of moderate to severe hidradenitis suppurativa in adult patients.1
ADULTS
INDUCTION
ADULTS
Day 1:
160 mg/0.8 mL*
>
Day 15:
80 mg/0.8 mL
MAINTENANCE
ADULTS
Day 29:
40 mg/0.8 mL
every other week
17 kg (37 lbs) to <40 kg (88 lbs)
Day 1:
80 mg/0.8 mL
>
Day 15:
40 mg/0.8 mL
Day 29:
20 mg/0.8 mL
every other week
≥40 kg (88 lbs)
Day 1:
160 mg/0.8 mL*
>
Day 15:
80 mg/0.8 mL
Day 29:
40 mg/0.8 mL
every other week
Current adalimumab patients do not need to repeat induction dose—initiate Adalimumab-fkjp as next scheduled dose.
*Given in 1 day or split over 2 consecutive days.
Indication: Adalimumab-fkjp is indicated for the treatment of moderately to severely active Crohn’s disease in adults and pediatric patients 6 years of age and older.1
INDUCTION
ADULTS
Day 1:
160 mg/0.8 mL*
>Day 15:
80 mg/0.8 mL
17 kg (37 lbs) to
<40 kg
(88 lbs)
Day 1:
80 mg/0.8 mL
>Day 15:
40 mg/0.8 mL
≥40 kg (88 lbs)
Day 1:
160 mg/0.8 mL*
>Day 15:
80 mg/0.8 mL
MAINTENANCE
ADULTS
Day 29:
40 mg/0.8 mL
every other week
17 kg (37 lbs) to
<40 kg
(88 lbs)
Day 29:
20 mg/0.8 mL
every other week
≥40 kg (88 lbs)
Day 29:
40 mg/0.8 mL
every other week
Current adalimumab patients do not need to repeat induction dose—initiate Adalimumab-fkjp as next scheduled dose.
*Given in 1 day or split over 2 consecutive days.
Indication: Adalimumab-fkjp is indicated for the treatment of moderately to severely active Crohn’s disease in adults and pediatric patients 6 years of age and older.1
ADULTS
INDUCTION
ADULTS
Day 1:
160 mg/0.8 mL*
>
Day 15:
80 mg/0.8 mL
MAINTENANCE
ADULTS
Day 29:
40 mg/0.8 mL
every other week
Current adalimumab patients do not need to repeat induction dose—initiate Adalimumab-fkjp as next scheduled dose.
Discontinue Adalimumab-fkjp in adult patients without evidence of clinical remission by 8 weeks (Day 57) of therapy.
*Given in 1 day or split over 2 consecutive days.
Indication: Adalimumab-fkjp is indicated for the treatment of moderately to severely active ulcerative colitis in adult patients.1
Limitations of Use: The effectiveness of adalimumab products has not been established in patients who have lost response to or were intolerant to TNF blockers.1
ADULTS
INDUCTION
ADULTS
Day 1:
80 mg/0.8 mL
MAINTENANCE
ADULTS
Day 8:
40 mg/0.8 mL
every other week
Current adalimumab patients do not need to repeat induction dose—initiate Adalimumab-fkjp as next scheduled dose.
Indication: Adalimumab-fkjp is indicated for the treatment of non-infectious intermediate, posterior, and panuveitis in adult patients.1
Rheumatology
Rheumatoid Arthritis
MAINTENANCE
40 mg/0.8 mL
every other week
Indication:
Psoriatic Arthritis
Content for Psoriatic Arthritis.
Ankylosing Spondylitis
Content for Ankylosing Spondylitis.
Juvenile Idiopathic Arthritis
Content for Juvenile Idiopathic Arthritis.
Uveitis
Content for Uveitis.
Dermatology
Dermatology Content
Gastroenterology
Gastroenterology Content
Ophthalmology
Ophthalmology Content
References: 1. Hulio. Product information. European Medicines Agency; 2022. Accessed March 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/hulio 2. Hulio. Product information. Health Canada; 2022. Accessed March 20, 2023. https://health-products.canada.ca/dpd-bdpp/info?lang=eng&code=99197 3. Data on file. Biocon Biologics Inc; 2023. 4. US Food and Drug Administration. Biosimilars: review and approval. 2022. Accessed April 24, 2023. https://www.fda.gov/drugs/biosimilars/review-and-approval 5. Adalimumab-fkjp. Prescribing Information. Biocon Biologics Inc; 2025. 6. HUMIRA. Prescribing information. AbbVie Inc; 2024.